How to Find Number of Electrons
The easiest way to find the number of protons neutrons and electrons for an element is to look at the elements atomic number on the periodic table. Then identify the charge of the ion which will be written as a superscript to the right of the element.

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets
In other words group number tells you the number of valence electrons of an atom.

. This means the number of electrons and the number of protons in an element are equal. Moreover since these two subatomic particles electrons and protons have opposite charges they. It carries a negative charge of 1602176634 1019 coulomb which is considered the basic unit of electric charge.
The oxidation states of iron are 2 and 3. Electron lightest stable subatomic particle known. One of the easiest ways to find valence electrons is by checking out the elements place in the periodic table.
There are a number of models to describe the hot-electron effect. The simplest predicts an electron-phonon e-p interaction based on a clean three-dimensional free-electron model. Of protons 17 of neutrons 37 17 20 of electrons 17 0 17 of protons 16 the atomic number is not given but can be found on the periodic table of neutrons.
Therefore the number of electrons in oxygen is 8. 500 pm - 930 pm dinner. 1130 am - 500 pm lunch.
An electron is therefore considered nearly massless in comparison with a proton or a neutron and the. Each orbital has its own set of quantum numbers such as energy. You can find the number of neutrons if you know the isotope of the atom.
Great lets apply the rules to some examples. The atomic number of an element is found through the number of protons present in the nucleus. Thus n1 shell can hold two electrons.
A subshell is a set of states which are defined by the total azimuth quantum number l in the shell. The wave-like behavior of a bound electron is described by a function called an atomic orbital. The easiest way to find the atomic mass of an element is to look on the periodic table.
1130 am - 500 pm lunch. That number is equal to the number of protons. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom.
The oxidation state of iron 2 has been used in the ironII oxide or ferrous oxideFeO. I bring thirty-two years of full-time classroom chemistry teaching experience and tens of thousands of hours of one-on-one chemistry tutoring across the globe to a seventeen year writing career that includes several best-selling international award-winning chemistry books and a burgeoning. The number of electrons in an unpaired state in the last shell after the electron configuration of an atom is called the valency of that element.
The rest mass of the electron is 91093837015 1031 kg which is only 11836the mass of a proton. First we look at the n1 shell the first shell. An s-orbital holds 2 electrons.
The 3d 4d etc can each hold ten electrons because they each have five orbitals and each orbital can hold two electrons 5210. Valency and valence electrons of iron. 500 pm - 1000 pm dinner.
The valence electrons of an element can be found by closely examining the vertical column in. How to find the Atomic Mass. The number of electrons in an atom is equal to the atomic number of an element for neutrally charged species.
Im a true chemistry freelancer and Subject Matter Expert SME. The results of his experiments show that each element has a unique integer equal to the number of positive charges in the nucleus of that element. Of neutrons mass number atomic number of electrons atomic number charge.
The number of protons is equal to the number of electrons unless theres an ion superscript listed after the element. How to easily find the number of electrons protons and neutrons in an oxygen atom. A neutral atom has the same number of protons and electrons charges cancel each other out.
To find the number of electrons an atom has start by looking up the element youre working with on the periodic table and locating its atomic number which will be in the upper left-hand corner of the square. An atom with an nth electron shell can hold 2n2 electrons which is the first shell that can hold 2 electrons the second shell can hold 8 electrons and so on. Build an Atom - PhET.
If the charge is negative electrons are in excess. If the charge is positive there are more protons than electrons. A system of one or more electrons bound to a nucleus is called an atom.
For example Let us consider group 1 of the Periodic table. Hot electrons arise generically at low temperatures even in degenerate semiconductors or metals. The group number tells you the total number of electrons present in the outermost orbit of an atom.
FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. If the number of electrons is different from the nucleuss electrical charge such an atom is called an ion. An ion has an unequal number of protons and electrons.
Thus to find the number of electrons possible per shell. Carbon has 6 protons in its nucleus making it also the sixth element in the periodic table. Scientist Henry Gwynn Jefferies Mosle examined the X-ray spectrum of various elements in 1913-to 1914.
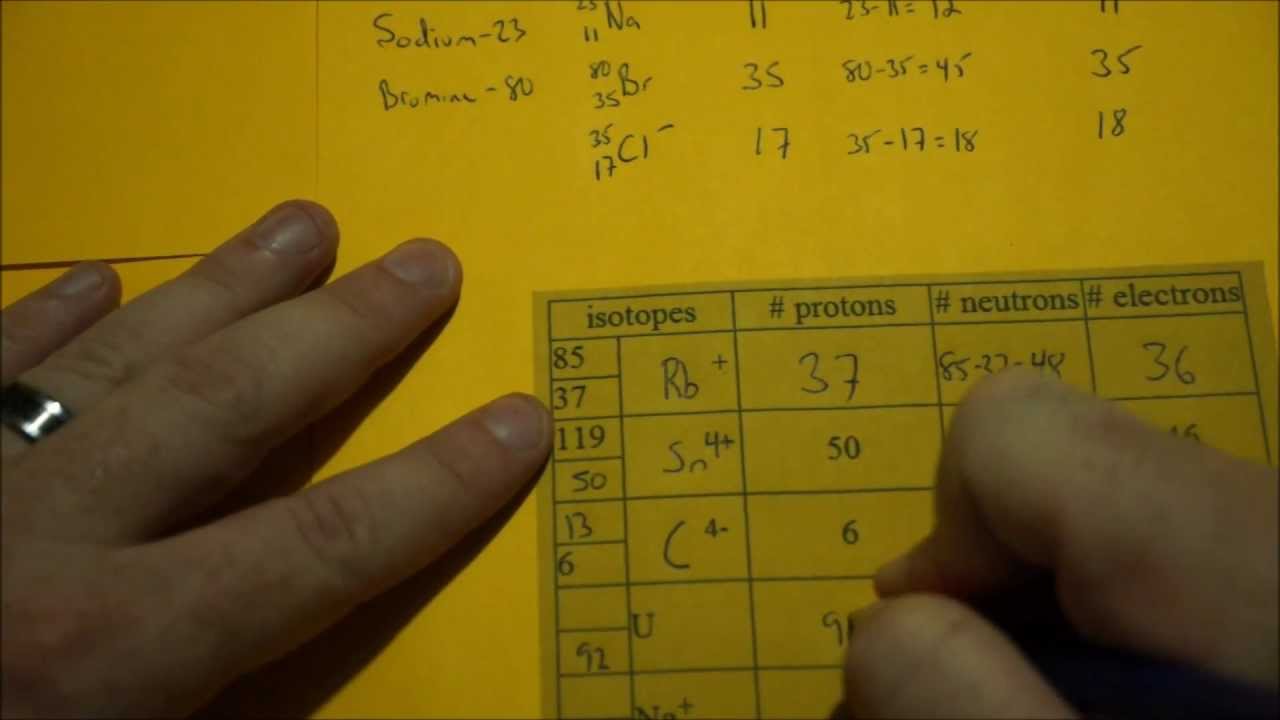
Determining Protons Neutrons And Electrons Atoms And Ions Protons Neutrons Electrons

How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Protons Proton Neutron Electron

Finding Protons Neutrons And Electrons Through The Atomic Number And Neutrons By Mass Atomic Proton Neutron Electron Protons Neutrons

No comments for "How to Find Number of Electrons"
Post a Comment